Tenecteplase (TNKase™):
A Clinical
Review
Volume IV, Number 1 | January/February 2001
Michael Militello, Pharm.D., BCPS
Return to Pharmacotherapy Update Index
Introduction
Fibrinolytic therapy is the pharmacologic modality of choice for patients presenting with ST-segment elevation myocardial infarction. Tenecteplase (TNK-tPA) is the newest fibrinolytic agent approved by the Food and Drug Administration. Tenecteplase is a 527 amino acid glycoprotein derivative of human tissue plasminogen activator that binds to fibrin and converts plasminogen to plasmin. Tenecteplase is a fibrin specific fibrinolytic. It is different from human tissue plasminogen activator by having three amino acid substitutions. These substitutions decrease plasma clearance, increase fibrin binding, and increase resistance to plasminogen activator inhibitor-1 (PAI-1). These changes in human tissue plasminogen activator increase plasma half-life and fibrin specificity and decrease the inactivation of tenecteplase by PAI-1. The modifications to tenecteplase confer the clinical advantages of tenecteplase by allowing the drug to be administered as a single intravenous bolus over 5 seconds and improving outcomes secondary to a more rapid clot lysis.
Pharmacokinetics
Tenecteplase has an initial half-life of 20 to 24 minutes with a terminal half-life of 90 to 130 minutes with a mean plasma clearance ranging from 99 to 119 mL/min. The initial volume of distribution approximates plasma volume and the major route of elimination is by hepatic metabolism. The area under the plasma concentration (AUC) curve of tenecteplase given as a single bolus is similar to the AUC obtained from a 90-minute infusion of tissue plasminogen activator (t-PA; alteplase).
Clinical Trials
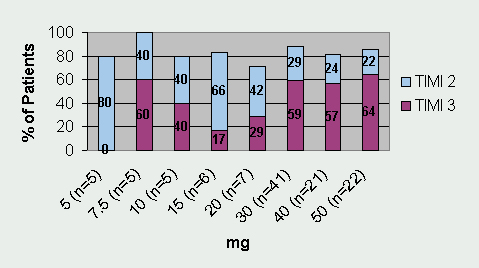
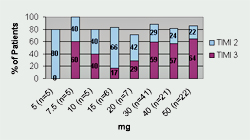
Tenecteplase has undergone three Phase II trials and one large Phase III trial. Thrombolysis In Myocardial Infarction 10A (TIMI 10A) trial was the first Phase II trial to use tenecteplase in the treatment of acute myocardial infarction. The authors evaluated doses ranging from 5- to 50-mg and collected data for the following: pharmacokinetics, coagulation parameters, TIMI grade 3 flow at 90 minutes, TIMI frame counting, bleeding complications, and allergic reactions. Patients (n=113) were randomized to receive one of eight doses of tenecteplase given as a single IV injection over 5 to 10 seconds. Results from this trial revealed that 30 mg and 50 mg of tenecteplase produced the greatest number of TIMI 3 flow rates at 90 minutes (See Figure 1). Serious bleeding occurred in 6.2% of the patients (n=7). Six of the bleeding events occurred at the catheterization access site and one bleeding complication occurred in a patient who underwent coronary artery bypass graft (CABG) surgery. There were no intracranial hemorrhages (ICH) or stroke in this small trial. The half-life of tenecteplase was also confirmed and is sufficiently long enough to consider it efficacious for single bolus dosing.
The second Phase II trial was Thrombolysis In Myocardial Infarction 10B (TIMI 10B) trial (n=886). This study was designed to compare angiographic efficacy and safety of tenecteplase 30- and 50-mg as a single bolus to front-loaded t-PA. The primary endpoint of this trial was TIMI grade 3 flow at 90 minutes. Secondary endpoints included: TIMI grade 3 flow at 60- and 75- minutes, TIMI grade 2 and 3 flow, TIMI frame counting, pharmacokinetic profiling, coagulation parameters, reinfarction, and serious bleeding. Early clinical data showed three events of ICH in the 50 mg tenecteplase group. The Safety and Monitoring Board recommended to suspend enrollment of this group. Additionally, the Board recommended that the dose of heparin be standardized and lowered based on the finding that those patients with ICH had higher than suggested doses of heparin. Heparin and aspirin were required adjunctive treatments; however, heparin dosing was at the discretion of the treating physician. The tenecteplase 50 mg dose was replaced with tenecteplase 40 mg, and there was a downward adjustment in heparin dosing.
After initiating a lower dose of tenecteplase and a heparin protocol, the incidence of intracranial and serious bleeding was lower in all treatment groups. TIMI grade 3 flow at 90 minutes was significantly less for the tenecteplase 30 mg group when compared to t-PA (p=0.035). However, the TIMI grade 3 flow rates for both the tenecteplase 40- and 50-mg groups, when compared to front-loaded t-PA, were no different. Safety data from this trial show that there was a higher percentage of ICH with high dose tenecteplase. The overall rates of ICH are listed in Table 1.
Table 1. Rates of Bleeding Complications in the TIMI 10B Trial
| Drug | n= | ICH (%) | Total Stroke (%) | Death (%) | Serious Bleeds (%) |
|---|---|---|---|---|---|
| Tenecteplase 30 mg | 308 | 1 | 1.9 | 3.6 | 1.9 |
| Tenecteplase 40 mg | 154 | 1.9 | 2.6 | 6.5 | 5.2 |
| Tenecteplase 50 mg | 78 | 3.8 | 5.1 | 3.8 | 11.5 |
| t-PA front-loaded | 316 | 1.9 | 2.8 | 5.7 | 8.5 |
A secondary analysis of the tenecteplase dose adjusted for weight revealed that those patients who received doses >0.55 mg/kg had higher rates of serious bleeding and ICH, regardless of what dose of heparin was administered. The analyses also included TIMI grade 3 flow rates based on weight adjustments. The authors found that the breakpoint for improved TIMI grade 3 flow was 0.5 mg/kg and doses below this point had significantly fewer patients achieving TIMI grade 3 flow.
Data from this trial suggest that tenecteplase at a dose of 0.5 mg/kg should allow similar efficacy and safety compared to front-loaded t-PA. Heparin dosing also appears to be of importance in decreasing the risk of bleeding complications. This trial was primarily designed to test efficacy whereas the ASSENT-1 trial (enrolling patients in parallel to TIMI 10B) was designed to evaluate the safety of a range of tenecteplase doses.
The final Phase II trial was the Assessment of Safety and Efficacy of a New Thrombolytic (ASSENT-1). This trial evaluated the single-bolus administration of tenecteplase in acute myocardial infarction and was conducted in parallel with the TIMI 10B trial. The ASSENT-1 trial was designed to evaluate the safety of tenecteplase 30- and 50-mg. Because of the 3.8% incidence of ICH in the TIMI 10B trial, the Data and Safety Monitoring Board recommended that the 50 mg dose of tenecteplase be replaced with 40 mg even though no patient in the ASSENT-1 trial who received 50 mg of tenecteplase had an ICH. Heparin adjustments also paralleled the TIMI 10B trial after the increased rates of ICH were learned. As with the other trials, all patients received 150 to 325 mg of aspirin.
The goal of the ASSENT-1 trial was to estimate the rate of ICH at 30 days with the different doses of tenecteplase. A historical control for t-PA was used in order to maximize the number of patients enrolled in the trial. There were 3301 patients enrolled in the study and 3235 patients received study drug.Reasons for patients not receiving drug (n=66) included: death before thrombolytic could be administered (n=2), decision not to give thrombolytic (n=29), and use of another thrombolytic (n=35). The compliance with other study medications was 96% or greater.
The rates of ICH and stroke were similar among all groups and less than what was predefined as unacceptable based on t-PA historical controls (See Table 2).
Table 2: Rates of Central Nervous System Events in the ASSENT-1 Trial
| TNK-tPA 30 mg | TNK-tPA 40mg | TNK-tPA 50mg | Total | |
|---|---|---|---|---|
| Total stroke | 26 (1.5%) | 22 (1.5%) | 0 | 48 (1.5%) |
| ICH | 16 (0.94%) | 9 (0.62%) | 0 | 25 (0.77%) |
| Ischemic stroke | 9 (0.5%) | 14 (1%) | 0 | 23 (0.7%) |
| Cerebral infarction with hemorrhagic conversion | 1 (0.1%) | 1 (0.1%) | 0 | 2 (0.1%) |
| Unknown type | 1 (0.1%) | 1 (0.1%) | 0 | 2 (0.1%) |
Because there was no comparative arm in ASSENT-1, the trial was not designed to show efficacy benefit. The safety data from ASSENT-1 and the efficacy data from TIMI-10B led to the Phase III ASSENT-2 trial.
Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) was the first Phase III trial for tenecteplase. This randomized, double-blind trial (n= 16,949) was designed to show equivalence of single-bolus weight adjusted tenecteplase to front-loaded weight adjusted t-PA (standard of care) in patients presenting with acute myocardial infarction (See Table 3).
Table 3: ASSENT-2: Dosing
| Patient Weight (kg) | Tenecteplase (mg) |
|---|---|
| <60 | 30 |
| 60-69 | 35 |
| 70-79 | 40 |
| 80-89 | 45 |
| greater than or equal to 90 | 50 |
Four hundred and forty-five patients did not receive study drug secondary to the following: detection of exclusion criteria after randomization (n=135), technical reasons (n=49), death or adverse events immediately after randomization (n=23), primary angioplasty (n=46), or open-labeled use of thrombolytics. The primary endpoint was all cause mortality at 30 days. Secondary endpoints included: death or nonfatal stroke at 30 days, major non-fatal cardiac events in hospital, and stroke. All stroke data were reviewed by an independent panel.
Data from the ASSENT-2 trial revealed that tenecteplase was equivalent to t-PA in patients with acute myocardial infarction. Mortality rates were not significantly different between the two groups, 6.179% mortality in the tenecteplase-treated group compared to 6.151% in the t-PA-treated group. Additionally, the Kaplan-Meier survival curves for the two treatment groups were similar. The rates of ICH were similar between tenecteplase and t-PA, 0.93% and 0.94%, respectively. The overall rates of stroke were also not significantly different. However, tenecteplase had a significantly lower incidence of bleeding complications as compared to t-PA, 26.43% versus 28.95%, respectively.
Also, patients who presented greater than 4 hours after the onset of symptoms had a significantly lower mortality rate when treated with tenecteplase compared to t-PA (7% and 9.4%, respectively; p=0.018). Although this was not a pre-specified endpoint, the data suggest that tenecteplase is more beneficial in patients who present greater than 4 hours, but less than 6 hours after the onset of symptoms.
A previous trial of reteplase and t-PA (GUSTO III) was designed to show superiority of reteplase over t-PA in patients presenting with acute myocardial infarction. In this trial, there were no significant differences in event rates between the two treatment groups. The data suggest that reteplase may be equivalent to t-PA; however, because the study was designed to prove superiority, it is not known if reteplase and t-PA are equally efficacious in treating ST-segment elevation myocardial infarction. The rate of ICH and stroke in the GUSTO III trial were similar between reteplase and t-PA. Also, the rates of major bleeding were similar between the two groups. The data to date from controlled trials prove that t-PA and reteplase are superior to streptokinase for the treatment of acute myocardial infarction. Reteplase is not superior to alteplase and based on previous study design, has yet to be proven equivalent. Tenecteplase has been shown to be equivalent to t-PA in the ASSENT-2 trial; however, there are no data comparing tenecteplase to reteplase at this time.
Contraindications
Similar to other thrombolytics, tenecteplase has the following contraindications: 1) active internal bleeding, 2) history of cerebrovascular accident, 3) intracranial or intraspinal surgery or trauma within 2 months, 4) intracranial neoplasm, 5) arteriovenous malformation, or aneurysm, 6) known bleeding diathesis, and 7) severe uncontrolled hypertension.
Warnings and Precautions
Similar to other thrombolytics, tenecteplase has the following warnings and precautions: 1) bleeding, 2) cholesterol embolization, 3) arrhythmias, 4) arterial and venous punctures should be minimized to avoid unnecessary bleeding, and 5) readministration of tenecteplase has not been evaluated and should be done with caution. Additionally, three of 487 patients tested for antibodies were positive at 30 days.
Adverse Reactions
The most frequent adverse event encountered in clinical trials was bleeding. Bleeding is divided into four categories: ICH, major bleeding, minor bleeding, and units of blood transfused. In the ASSENT-2 trial with nearly 17,000 patients enrolled, the rates of major and minor bleeding as well as blood transfusions were significantly lower with tenecteplase than with t-PA. The rates of ICH were the same between the two treatment groups (0.9%). The incidence of ICH for reteplase in GUSTO III (0.91%) was similar to that seen with tenecteplase in the ASSENT-2 trial. The overall incidence of stroke in the ASSENT-2 trial was 1.8% and 1.7% in the tenecteplase and t-PA groups, respectively. These rates are similar to published data for reteplase.
Other adverse events in which causality could not be established include: allergic reactions (<1%), anaphylaxis (<0.1%), cardiogenic shock, arrhythmias, atrioventricular block, pulmonary edema, heart failure, cardiac arrest, recurrent myocardial infarction or ischemia, myocardial rupture, cardiac tamponade, pericarditis, pericardial effusion, mitral regurgitation, thrombosis, embolism and electromechanical disassociation.
Drug Interactions
Formal drug interaction studies have not been performed with tenecteplase. All patients in the clinical trials received aspirin (150 to 325 mg) and heparin. Glycoprotein IIb/IIIa inhibitors, warfarin, dipyridamole and adenosine diphosphate inhibitors may increase the risk of bleeding if administered prior to, during, or after tenecteplase administration.
Recommended Monitoring
The most common adverse reaction of thrombolytic therapy is bleeding. Therefore, patients should have a baseline complete blood count with periodic monitoring similar to other thrombolytics. Also, because there is a risk of stroke and ICH, mental status should be routinely evaluated for the first 24 hours after administration of tenecteplase. There is a small risk of allergic reaction, therefore, medications to treat anaphylaxis should be readily available.
Dosage and Administration
Tenecteplase is dosed based on weight and is given as a single-bolus injection over 5 seconds (See Table 4). This drug is not compatible with dextrose, and therefore, should not be given in the same intravenous line. Lines containing dextrose should be flushed before and after administration.
Table 4: Weight Adjusted Dosing
| Patient Weight (kg) | Tenecteplase (mg) | Volume of Tenecteplase to be administered (ml) |
|---|---|---|
| <60 | 30 | 6 |
| 60-69 | 35 | 7 |
| 70-79 | 40 | 8 |
| 80-89 | 45 | 9 |
| greater than or equal to 90 | 50 | 10 |
Product Availability
Tenecteplase is supplied as a sterile, lyophilized powder in a 50 mg vial. Each 50 mg vial of tenecteplase is packaged with one 10 ml vial of sterile water for injection, USP for reconstitution. Vials of tenecteplase contain no preservatives and must be used within 8 hours of reconstitution. The cost is $2,200 for each 50 mg vial.
Conclusion
Tenecteplase, a fibrin specific thrombolytic with a sufficiently long half-life to enable single bolus administration, has proven to be as efficacious as t-PA in reducing mortality rates in patients presenting with AMIs. There were similar stroke and ICH events between each group; however, there were fewer bleeding events overall in the tenecteplase-treated patients. The advantage of this agent is its ability to be given as a single 5 to 10 second bolus, thus eliminating the need to adjust the infusion rate as with t-PA or timing the second dose of reteplase to be 30 minutes after the first dose.
References Available Upon Request