Bosentan (Tracleer™), a New Agent
for the
Treatment of
Pulmonary
Arterial Hypertension
Volume V, Number 2 | March/April 2002
Andreea Popa, Pharm.D. Candidate
Return to Pharmacotherapy Update Index
Introduction
Pulmonary
arterial hypertension (PAH) is a devastating disease defined by an increase
in pulmonary vascular resistance which leads to right ventricular failure
and ultimately to death.1 Vasoconstriction has been noted to
be part of the etiology of this disease.2 The release of locally
active endothelial vaso-proliferative and vasoconstrictive substances
leads to pulmonary vascular remodeling and progression to vascular obstruction.3
Pulmonary arterial
hypertension (PAH) can be classified into two categories: 1) primary pulmonary
hypertension (PPH), a primary idiopathic disease, or
2) PAH secondary to collagen vascular diseases, congenital systemic to
pulmonary shunts, portal hypertension, HIV infection, drugs, and pulmonary
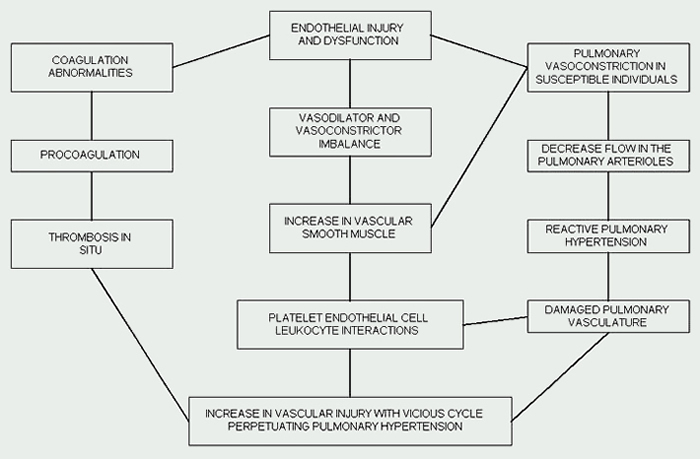
hypertension of the newborn See Figure 1.3
Even though the etiology of PAH may be different, the therapeutic goal for all patients is similar.3 Current medical management of PAH (See Table 1) includes vasodilators, anticoagulants, inotropic agents, diuretics and oxygen. Vasodilators, such as calcium channel blockers and prostacyclin (PGI2), are used to reduce pulmonary arterial pressure and increase cardiac output. Calcium channel blockers are effective in 20% of adults and 40% of children, and are not effective in patients which have failed acute vasodilator testing.
Table 1. Summary of Conventional Therapies in PAH4
| Treatment Measure | Intervention | Comments |
|---|---|---|
| Physical activity | Encouraged to avoid sedentary lifestyle due to muscular deconditioning. | Watch for anginal chest pain and syncope. |
| Avoidance of aggressions | Avoid acute stresses and invasive medical procedures. | Due to an increase in sympathetic nervous system activation, an increase in PVR is poorly tolerated. |
| Avoid pregnancy | Low-dose hormonal contraception. | |
| Avoid hypoxia | Avoid high altitudes; treat pulmonary infections. | Due to superimposed hypoxic pulmonary vasoconstriction, hypoxia aggravates PAH. Supplemental oxygen rarely indicated. |
| Avoid appetite-suppressant drugs | Absolute contraindication. Agents include dexfenfluramine. | These agents are known to cause PAH. |
| Anticoagulation | Maintain an INR between 2 to 3 with warfarin (Coumadin®). | These patients are at risk for venous thromboembolism. |
| Diuretics | Various doses are required depending on volume status of the patient. | Diuretics optimize preload by adjusting volume status and venous return. |
| Inotropes | Agents include dobutamine, dopamine (Inotropin®), and digoxin (Lanoxin®). | To increase contractility; RV contractility is usually preserved in these patients. |
| Vasodilators | •Calcium channel blockers:
•Epoprostenol 2 ng/kg/min and increased by 2 ng/kg/min every 10 to 15 minutes, as tolerated (see text). |
Vasodilators decrease afterload by decreasing PVR. |
PVR: peripheral vascular resistance, INR: international normalized ratio, RV: right ventricular
Nonresponders are usually treated with a continuous infusion of prostacyclin, such as epoprostenol (Flolan®; GlaxoSmithKline), through a permanent central venous line. Epoprostenol therapy is technically demanding and expensive. It requires the maintenance of an indwelling infusion catheter and ambulatory infusion pump. Epoprostenol is light and temperature sensitive, therefore it requires constant cooling and protection from light. Reconstitution must be done only with the diluent supplied by the manufacturer.
Abrupt interruptions or large, sudden reductions in epoprostenol dosage may lead to rebound pulmonary hypertension. Non-cardiogenic pulmonary edema may develop during dose ranging.
Some of the most common adverse reactions to epoprostenol therapy include jaw pain, flushing, heart failure, shock, syncope, tachycardia, anxiety, chills, dizziness, fever, headache, hyperesthesia, nervousness, pain, diarrhea, nausea, vomiting, myalgias, hypoxia, and flu-like symptoms.
Epoprostenol therapy requires acute dose ranging followed by a continuous infusion. During acute dose ranging, the initial infusion rate is 2 ng/kg/min, and it is increased by 2 ng/kg/min every 15 minutes until dose-limiting side effects occur. The epoprostenol continuous infusion is started initially at 4 ng/kg/min less than the maximum tolerated dose during the acute dose ranging. Dose adjustments during treatment are based on the worsening of symptoms and dose-limiting side effects. Close monitoring during dose ranging and dose adjustments is necessary.
Because these patients are at an increased risk for thrombotic events, anticoagulation therapy is utilized.3,4 Endothelial injury and dysfunction accompanied by coagulation abnormalities often play a role in the pathogenesis of PAH. The increase in vascular injury and the decrease in the cross-sectional area of the pulmonary vascular bed places patients at a high risk for micro-vascular thrombosis.3,4
Bosentan
The Food and Drug Administration (FDA) approved bosentan (Tracleer™; Actelion Pharmaceuticals US, Inc.) in November 2001 for the treatment of PAH, to improve exercise ability and decrease the rate of clinical worsening in patients with World Health Organization (WHO) class III or IV symptoms (See Table 2).Bosentan is an orally active, nonpeptide, competitive antagonist of both ETA and ETB (endothelin type A and B) receptors, with a slightly higher affinity for the ETA receptor. Bosentan competes with Endothelin-1 (ET-1), a neurohormone that binds at the ETA and ETB receptors, leading to the constriction of the pulmonary arteries when it binds to ETA receptors and vasodilatation when it binds to ETB receptors.5 Concentrations of ET-1 are elevated in the plasma and lung tissue of PAH patients, therefore suggesting a pathogenic role of ET-1 in this disease.7
Table 2. WHO Functional Assessment for Pulmonary Hypertension
(modified after NYHA functional classification)6
| WHO Class | Description |
|---|---|
| Class I | Patients with pulmonary hypertension but without resulting limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain or near syncope. |
| Class II | Patients with pulmonary hypertension resulting in a slight limitation of physical activity. They are comfortable at rest. Ordinary physical activity causes undue dyspnea or fatigue, chest pain or near syncope. |
| Class III | Patients with pulmonary hypertension resulting in marked limitation of physical activity. They are comfortable at rest. Less than ordinary activity causes undue dyspnea or fatigue, chest pain or near syncope. |
| Class IV | Patients with pulmonary hypertension with inability to carry out any physical activity without symptoms. These patients manifest signs of right heart failure. Dyspnea and/or fatigue may even be present at rest. Discomfort is increased by any physical activity. |
Pharmacokinetics5,8
Bosentan is 50% bioavailable and 98% bound to plasma proteins with an elimination half-life of 5 hours. It reaches maximum plasma concentration within 3 to 5 hours following oral administration. Bosentan is metabolized by and is an inducer of the cytochrome (CYP) P450 isoenzymes 2C9, 3A4 and possibly 2C19; therefore, bosentan induces its own metabolism. It is metabolized to three metabolites, of which one is active and eliminated by biliary excretion following hepatic metabolism. This active metabolite may contribute 10 to 20% of the effect of bosentan.
Selected Bosentan Clinical Trials (Table 3)
Rubin1 and colleagues conducted a randomized, double-blind, placebo-controlled, multicenter trial in 213 patients with PAH, mostly PPH with WHO class III or IV despite treatment with anticoagulants, vasodilators, inotropes, or oxygen. The bosentan regimen was 62.5 mg orally twice daily for 4 weeks, then 125 mg orally twice daily or 250 mg orally twice daily for 12 additional weeks. Exercise capacity at week 16, measured by the 6-minute walk test, was the primary end point of the study. The 6-minute walk increased by 27 meters (m) (326 to 353 m) in the bosentan group (p=0.01), while there was no change in the placebo group (344 to 336 m). The patients on the 250 mg twice daily dose experienced a greater increase in the 6-minute walk test (333 to 379 m). Overall, 9% of the treatment group experienced abnormalities in their hepatic function (4% in the 125 mg twice daily group and 14% in the 250 mg twice daily group). An increase in aminotransferases of more than eight times the upper limit was noted in two patients receiving 125 mg twice daily and 5 patients receiving 250 mg twice daily. None of the patients in the placebo group experienced any abnormalities in their hepatic function tests.
Channick13 and colleagues conducted a randomized, double-blind, placebo-controlled, multicenter trial in 32 patients with PAH, mostly PPH regardless of treatment with vasodilators, anticoagulants, diuretics, inotropes, or oxygen. The bosentan regimen was 62.5 mg orally twice daily for 4 weeks, then 125 mg orally twice daily, if tolerated, for up to 12 weeks, with an extension to 28 weeks in some patients. Exercise capacity at week 12, measured by the 6-minute walk test, was the primary end point of the study. The 6-minute walk test increased by 71 m at 12 weeks (360 to 430 m) in the bosentan group (p<0.05), while there was no change in the placebo group (355 to 349 m). Nine of the 21 patients in the bosentan group improved to WHO class II while the rest remained WHO class III. Adverse events were transient and similar between the two groups. Two patients in the bosentan group experienced a transient increase in transaminases. There were no changes in hematological or biochemical parameters, nor were there reports of hypotension.
At the American College of Cardiology (ACC) meeting in March 2002, Packer and colleagues presented the results of the ENABLE trial. This trial enrolled 1,613 patients with severe CHF already on conventional treatment and NYHA class IIIb or IV. The bosentan regimen was 500 mg orally twice daily, then 125 mg orally twice daily for approximately 18 months. The primary outcome of the study was all-cause mortality and hospitalization for heart failure. Statistical significance was not reached for the primary end point of risk reduction in time to death or hospitalization due to heart failure. Transient worsening of heart failure was seen in patients when bosentan was started and patients developed immediate and sustained fluid retention. Further data from this trial will provide more insight into the safety and tolerability of bosentan.
Warnings/Precautions5
Bosentan is rated as a pregnancy-risk category X. Pregnancy-risk category X is defined as: studies in animals or humans have demonstrated fetal abnormalities or there is evidence of fetal risk based on human experience, or both, and the risk of the use of the drug in pregnant women clearly outweighs any possible benefit. Therefore, a pregnancy-risk category X drug is contraindicated in women who are or may become pregnant. Impairment of fertility/testicular function and potential for birth defects was observed in animal studies. The most severe adverse effects of bosentan include liver toxicity (a 3-fold increase in aminotransferase enzymes, AST-aspartate aminotransferase/ALT-alanine aminotransferase, in 11% of patients) and a dose-related decrease in hemoglobin (6%) and hematocrit. Baseline and monthly pregnancy tests are required in female patients along with close monitoring of liver enzymes, hemoglobin, and hematocrit in all patients. Other common side effects during treatment were headache (16%), flushing (7%), leg edema (5%) and anemia (3%).
Monitoring5
Pregnancy must be excluded before starting treatment with bosentan. Oral, injectable, and implantable estrogen/progesterone contraceptives should not be used as the sole method of contraception. Plasma levels of oral, injectable, and implantable estrogen/progesterone contraceptives are likely to be decreased due to the inducible effects of bosentan on CYP3A4, the most common route of metabolism for these agents.
Liver aminotransferases should be obtained at baseline, and then, monthly after initiation of treatment. Adjustments in dosage are done according to AST/ALT levels (See Table 5). If clinical symptoms accompany the rise in AST/ALT levels, bosentan should be discontinued.
Hemoglobin levels should be monitored at 1 and 3 months, and every 3 months thereafter. Decreases in hemoglobin were noticed during the first few weeks of treatment.
Drug-Drug Interactions5
Pharmacokinetic studies have revealed that bosentan is an inducer of CYP2C9 and CYP3A4. This leads to an array of potential drug-drug interactions. Failure of oral, injectable, and implantable estrogen/progesterone contraceptives is anticipated, therefore an alternative method of contraception should be used. Bosentan, by being an inducer of CYP3A4, will induce the metabolism of oral, injectable, and implantable estrogen/progesterone contraceptives; therefore, reducing their plasma concentration (See Table 4).
Table 4. Bosentan Drug-Drug Interactions5
| Bosentan Drug Interactions | Comments |
|---|---|
| Cyclosporine |
•Cyclosporine
causes a 3- to 4- fold increase in bosentan steady state concentrations
( via CYP3A4). |
| Glyburide (DiaBeta®, Micronase ®, Glynase™) |
•Bosentan decreases glyburide plasma concentrations by ~40% (via CYP2C9 and CYP3A4). |
| Ketoconazole (Nizoral®) |
•Ketoconazole causes a 2-fold increase in bosentan plasma contraindications (via CYP3A4).
•No bosentan dose adjustment is necessary; but increased side effects should be considered. |
| HMG-CoA reductase inhibitors |
•Bosentan decreases
simvastatin plasma concentrations by ~50% (via CYP3A4)
• Bosentan is expected to decrease plasma concentrations of lovastatin, atorvastatin and other HMG-CoA reductase inhibitors metabolized via CYP3A4. • Reduced HMG-CoA reductase inhibitor efficacy should be considered; lipid profile should be monitored; HMG-CoA reductase inhibitor dose should be adjusted accordingly. |
| Oral, injectable, and implantable contraceptives |
•Bosentan is predicted
to decrease levels of oral, injectable and implantable estrogen/progesterone
contraceptives (via CYP3A4).
• Women should not rely on hormonal contraception alone when taking bosentan. |
| Warfarin15 (Coumadin® |
•Bosentan decreases plasma concentrations of S-warfarin (CYP2C9) and R-warfarin (CYP3A4)
by 29 and 38%, respectively.
• Clinical experience showed no clinically relevant changes in INR. • INR should be monitored closely. |
Dosage and Administration5
Bosentan treatment should be initiated at 62.5 mg administered orally twice daily for 4 weeks, and then, increased to a maintenance dose of 125 mg administered orally twice daily. In patients that develop aminotransferase elevations, the dose of bosentan should be adjusted according to the ALT/AST levels (See Table 5).
The bosentan dose does not need to be adjusted in renal insufficiency. In patients that have a low body weight (< 40 kg), but are > 12 years of age, the recommended initial and maintenance dose is 62.5 mg administered orally twice daily.
Table 5. Dosage Adjustment and Monitoring in Patients Developing Aminotransferase Abnormalities5
| ALT/AST levels | Dosage recommendations |
|---|---|
| > 3 and < 5 x ULN | Confirm and reduce daily dose or interrupt treatment. Monitor ALT/AST every 2 weeks. If levels return to pretreatment levels, continue or reintroduce treatment at starting dose. |
| > 5 and < 8 x ULN | If confirmed, stop treatment. Monitor ALT/AST every 2 weeks. If levels return to pretreatment levels, reintroduce treatment at starting dose. |
| > 8 x ULN | Stop treatment. Reintroduction should not be considered. |
ULN: Upper Limit of Normal
Cost
The average wholesale price is $49.50 per day for both 62.5 mg and 125 mg tablets and $2,970 per month.17
Bosentan distribution is only through a direct distribution program due to the black box warnings of liver toxicity and damage to the fetus.5 Prescribers should contact the Tracleer™ access program, at 1-866-228-3546, to obtain a Tracleer™ patient enrollment form. The completed Tracleer™ patient enrollment form needs to be faxed to 1-866-279-0669. Copies of the Tracleer™ patient enrollment form are available from the CCF Drug Information Center. Tracleer™ patient assistance program is also available from Actelion Pharmaceuticals US, Inc.
Summary
Bosentan represents a new development in the treatment of pulmonary hypertension, a different approach to the pathogenesis of this disease. Further trials are needed to assess bosentan's exact place in the pharmacotherapy of PAH and the patient population that would benefit most (e.g., patients with cardiogenic PAH were excluded from most of the trials).
A difference in the 6-minute walk test was noted at 1 month and became statistically significant at 2 months in the studies, therefore orally administered bosentan does not constitute an acute treatment for PAH.
At this time, bosentan should not be considered first-line therapy for PAH, and the current regimens (e.g., calcium channel blockers, inotropes, diuretics, oxygen and anticoagulants) should still be initiated first. It might be considered in a patient before going to continuous infusion of epoprostenol. Bosentan has not yet been requested or reviewed for addition to the formulary at The Cleveland Clinic Foundation.