Dofetilde: A New Class III
Antiarrhythmic
Volume III, Number 4 | September/October 2000
Jodie Zalewski, Pharm.D.
Return to Pharmacotherapy Update Index
Introduction
Dofetilide (Tikosyn®) is a new class III antiarrhythmic agent used for the conversion to and maintenance of normal sinus rhythm in patients with highly symptomatic atrial fibrillation/flutter. It is different from other class III antiarrhythmics in that it exhibits selective potassium channel blockade, with no effect on the conduction system. Dofetilide prolongs both atrial and ventricular repolarization, and therefore increases the refractory period within the cardiac muscle. Other class III antiarrhythmic agents possess additional antiarrhythmic properties. Sotalol (a ß-blocker) and amiodarone decrease AV nodal conduction. For this reason, coupled with the organ toxicities of amiodarone (e.g. pulmonary fibrosis, thyroid and hepatic dysfunction), these agents are not suitable for all patients. The selective mechanism of dofetilide appears to be better than other agents in its class; however, dofetilide must still be used with caution due to its adverse effects.
Adverse Effects
Because dofetilide acts on the potassium channel and widens the action potential, prolongation of the QT interval occurs, which is associated with torsades de pointes. Prolongation of the QT interval is directly related to the dose and plasma concentration of dofetilide. The concentration may become elevated due to drug interactions or poor renal function. Therefore, dofetilide is reserved for use only in highly symptomatic patients.
The FDA has mandated the manufacturer of dofetilide, Pfizer, to impose strict guidelines to assure appropriate use and correct dosing. The impetus was the heightened awareness of the potential for drug-induced torsades de pointes (demonstrated by the removal of astemizole, terfenadine, and cisapride from the market). In order for dofetilide therapy to be initiated, a patient must be admitted to a certified hospital for at least 72 hours for cardiac monitoring. Before a hospital is a certified facility, a form verifying the completion of Pfizer's education program by staff members must be on file with the company.
Patient Assessment
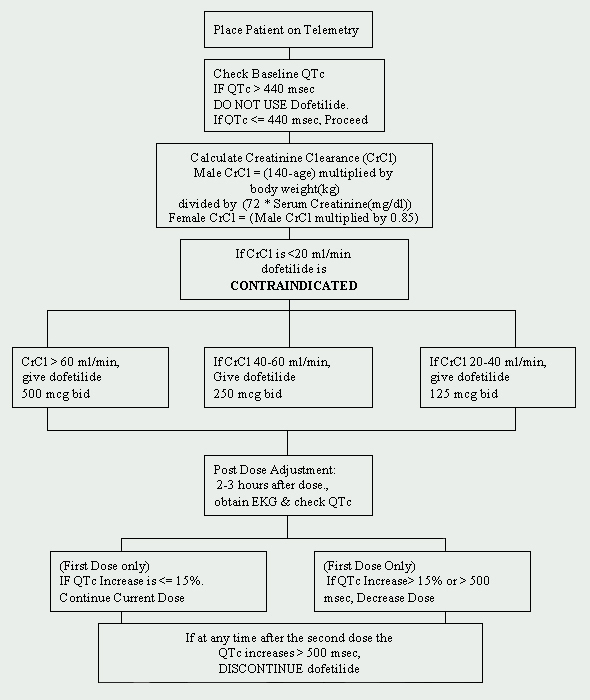
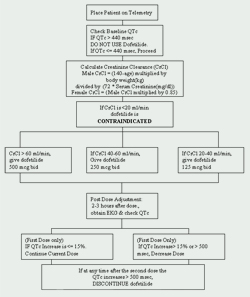
At the Cleveland Clinic Foundation, dofetilide is restricted to the Department of Cardiology. A protocol for the initiation of dofetilide has been implemented and is in compliance with the manufacturer's imposed regulations (See Figure 1). Patients to be started on dofetilide must be admitted to an approved telemetry unit for electrocardiographic (EKG) monitoring. Such monitoring is necessary to determine the presence of QT prolongation. Baseline QTc (corrected QT interval: QTc = QTMsec / square root of RR sec ) measurements are required and if the QTc is greater than 440Msec (500 Msec in patients with ventricular conduction abnormalities), dofetilide is contraindicated.
Another crucial baseline assessment is renal function. Because dofetilide is renally eliminated, poor renal function (measured by creatinine clearance [CrCl]) can lead to the accumulation of dofetilide, and therefore, increase the risk for arrhythmias. The initial dose of dofetilide is determined by the creatinine clearance (See Table 1). Patients with normal renal function (CrCl > 60 ml/min) are initiated on 500 mcg administered orally twice daily. Dofetilide is contraindicated in patients with a creatinine clearance of < 20 ml/min.
Table 1: Initial Dofetilide Dose
| Calculated CrCl (ml/min) | Dofetilide Dose |
|---|---|
| >60 |
500 mcg twice daily |
| 40-60 | 250 mcg twice daily |
| 20-39 | 125 mcg twice daily |
| <20 |
125 mcg twice daily |
A baseline basic metabolic panel (BMP) should also be obtained. Electrolyte abnormalities must be corrected before dofetilide is initiated because hypokalemia and hypomagnesemia may make patients more susceptible to arrhythmias. If serum potassium (K+) is < 4 mEq/L and/or the serum magnesium (Mg++) is < 2 mEq/L, then deficiencies must be repleted and rechecked before dofetilide is administered.
Drug interactions must also be addressed before beginning dofetilide therapy. Concomitant use of verapamil (Calan®, Calan SR®, Covera-HS®, Isoptin®, Isoptin SR®, Verelan®, Verelan PM®), cimetidine (Tagamet®), trimethoprim (Proloprim®, Trimpex®, or in combination products Bactrim®, Septra®), ketoconazole (Nizoral®), prochlorperazine (Compazine®) and megestrol (Megace®) with dofetilide is contraindicated. These agents slow the elimination of dofetilide by inhibiting the renal transport system, which leads to an increase of dofetilide levels. In addition, drugs that do not inhibit, yet are otherwise secreted via this route should be used with caution (e.g., triamterene, metformin, amiloride). Other agents that should be avoided are those that prolong the QT interval, such as phenothiazines, cisapride, bepridil, tricyclic antidepressants, erythromycin, and class I (e.g., quinidine, procainamide, lidocaine, flecainide) and class III antiarrhythmic agents. Lastly, agents that cause hypokalemia or hypomagnesemia, such as diuretics, should also be used with caution.
Dosing, Administration and Monitoring
If the patient is devoid of all contraindications, dofetilide may be initiated. A repeat EKG is performed 2 to 3 hours after the first dose to assess the QTc interval. If the QTc interval has increased > 15% or if the QTc is > 500Msec (550Msec in patients with ventricular conduction abnormalities), the dose of dofetilide must be decreased. In general, the dose is halved (See Table 2). For the rest of the 72-hour initiation period, EKG monitoring is performed at two to three hours after each subsequent dose of dofetilide. No additional dose reductions are recommended based on QTc interval. If at any time after second dose the QTc becomes > 500Msec (550 Msec in patients with ventricular conduction abnormalities), dofetilide should be discontinued. Continuous cardiac monitoring by telemetry is continued for a minimum of 72 hours, or 12 hours after electrical or pharmacological conversion to normal sinus rhythm, whichever is greater.
Table 2: QT Prolongation Adjustment Table
| Starting Dose Based on CrCl | Adjusted Dose (for QT Prolongation) |
|---|---|
| 500
mcg twice daily |
250 mcg twice daily |
| 250 mcg twice daily | 125 mcg twice daily |
| 125 mcg twice daily | 125 mcg once daily |
Pfizer, in conjunction with the FDA, has taken another step to assure proper use of dofetilide. Only one pharmacy (mail-order) in the United States, Statlanders Pharmacy in Pennsylvania, dispenses dofetilide. A Pharmacy Services Enrollment form must be completed and faxed to Statlanders prior to patient discharge to prevent disruption of therapy. Upon discharge, patients receive a one-week supply of drug. This supply gives the patient enough medication until their mail order prescription is delivered.
Conclusion
Although dofetilide is an effective antiarrhythmic agent for symptomatic atrial fibrillation/flutter, it must be initiated with caution. Strict adherence to the FDA-mandated guidelines is necessary to ensure patient safety.
References Available Upon Request