5-HT3 Receptor Antagonists
and ECG Effects
Volume V, Number 6 | November/December 2002
Mandy C. Leonard, Pharm.D., BCPS
Michael L. Militello, Pharm. D., BCPS
Return to Pharmacotherapy Update Index
Introduction
The 5-HT3 receptor antagonists have resulted in a major advancement in the management of chemotherapy-induced (CINV), radiation-induced (RINV), and post-operative nausea and vomiting (PONV), as they exhibit a high level of antiemetic activity with a low incidence of adverse effects. Comparative clinical trials among the three agents have not demonstrated any clinically relevant differences in efficacy or safety for the prevention of CINV or PONV. However, questions have been raised about minor electrocardiographic changes caused by each of the currently available 5-HT3 receptor antagonists.
Nausea & Vomiting
Many patients experience CINV, RINV, and PONV.
Nausea is defined as a subjectively unpleasant sensation associated with flushing, tachycardia, and an awareness of the urge to vomit.
Vomiting is the contraction of the abdominal muscles, descent of the diaphragm, and opening of the gastric cardia, resulting in the expulsion of stomach contents from the mouth.
Retching is defined as a spasmodic contraction of the diaphragm, thoracic and abdominal walls without expulsion of gastric contents.
The incidence of CINV varies depending on the emetogenic potential of the chemotherapy. Nausea and vomiting associated with radiation is dependent on radiation field size, site, and dose per fraction. And the incidence of PONV depends on multiple risk factors, such as type of surgery, sex, and concomitant medications.
Pharmacotherapy
Because of the potentially serious nature of CINV, RINV, and PONV, extensive research has been undertaken to find successful approaches for prevention and treatment. The 5-hydroxytryptamine type three (5-HT3) receptor antagonists are effective therapies for the management of CINV, RINV, and PONV and offer significant benefits over traditional treatments such as phenothiazines (eg, prochlorperazine [Compazine®], promethazine [Phenergan®]), benzamides (eg, metoclopramide [Reglan®]), trimethobenzamides (Tigan®), butyrophenones (eg, haloperidol [Haldol®], droperidol [Inapsine®]), and benzodiazepines (eg, lorazepam [Ativan®]). Currently, there are three 5-HT3 receptor antagonists available in the United States: dolasetron (Anzemet®), granisetron (Kytril™), and ondansetron (Zofran®).
Each agent is approved for the prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy and for the prevention of PONV. Additionally, no 5-HT3 receptor antagonist is currently indicated for treating CINV; however, dolasetron and granisetron are approved for treating PONV. Granisetron and ondansetron are indicated for the prevention of RINV.
Members of this class produce their antiemetic effects by selectively blocking 5-HT3 receptors in the gut and the brain stem, receptors that, when stimulated, lead to initiation of the emetic response.
Safety Profiles
The 5-HT3 receptor antagonists are well tolerated. The most common adverse events experienced by patients are headache, diarrhea, constipation, and fever. The 5-HT3 receptor antagonists are not associated with sedation or extrapyramidal side effects. Furthermore, the 5-HT3 receptor antagonists exhibit no significant drug interactions with common anesthetic agents and have little or no affinity for receptor sites other than the 5-HT3 receptors.
Although the 5-HT3 receptor antagonists have favorable and similar safety profiles, some minor ECG changes have been reported with this class of agents, including prolongations of the PR interval, the QRS complex, and the QT interval in healthy volunteers and in patients undergoing cancer chemotherapy or surgery. These potential changes have been the source of some concern with regard to their clinical significance.
When evaluating literature regarding the 5-HT3 receptor antagonists and ECG effects, it is important to understand the cardiac action potential and ECG parameters.
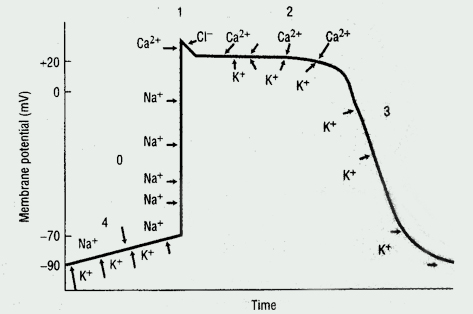
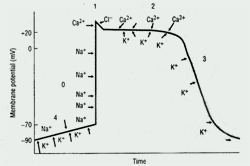
Cardiac Action Potential
The cardiac action potential, the basic unit of electrical activity in the heart, produces cardiac contractions. Cardiac myocytes, like other types of muscle cells, have a negative potential difference (-90 mV) at rest between the cell membrane and extra-cellular space (ie, they are polarized). Under the influence of trigger events, potassium, sodium, and calcium ions cross the cell membrane, thereby generating discrete ion currents (See Figure 1).
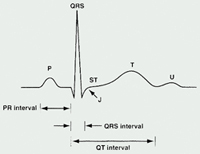
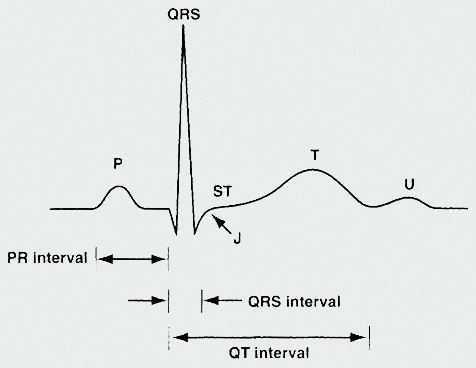
Electrocardiogram
The ECG represents a summation of action potentials across the entire heart. The ECG reading includes interpretation of the following: P wave, PR interval, QRS complex, QT interval, and ST segment. (See Table 1 and See Figure 2).
Table 1. ECG Parameters
| Parameter | Measures [normal range in milliseconds (ms)] |
|---|---|
| P Wave | Depolarization of the atria |
| PR Interval | Time from
the onset of atrial depolarization (P wave) to onset of ventricular depolarization
(QRS complex) Duration of atrioventricular conduction [120 to 200] |
| QRS Complex | Duration of depolarization of the ventricles [<120] |
| QT Interval | Depolarization
and repolarization of the ventricles [<420; potentially <440 in women] |
| RR Interval | Duration of ventricular cardiac cycle (an indication of ventricular rate) |
The QT interval and corrected QT interval (QTc)
Torsades de pointes. Even a small change in ion currents may be detrimental to the cardiac myocytes and may produce cardiac arrhythmias. If the action potential is prolonged during the repolarization phase, the membrane potential may abruptly reverse course and depolarize instead. An early after-depolarization may occur resulting in a second action potential, which has the potential to produce ventricular arrhythmias. Torsades de pointes ("twisting of the points"), a potentially life-threatening, polymorphic ventricular arrhythmia, may develop if this process is repetitive and self-sustaining.
Congenital or acquired prolongation of the QT interval has been associated with a risk of developing torsades de pointes, and there is some evidence that QT interval prolongation may also be a risk factor for sudden death. Other risk factors for torsades de pointes include electrolyte imbalance, bradycardia, and certain medications.
What is a normal QTc interval?
Interpretation of the QT interval can be challenging since QT varies inversely with heart rate (ie, QT interval decreases as heart rate increases). Consequently, the corrected QT interval (QTc) is used when heart rate exceeds 60 beats per minute (bpm) to investigate possible effects of treatment or pathologic processes on the intrinsic duration of the QT interval. Bazett's formula calculates a QTc that accountsfor individual variations in heart rate and is useful when the heart rate is >60 bpm:
QTc = QT/(RR)0.5
(where RR is the mean interval in
seconds between QRS complexes)
A QTc interval of 440 ms is considered by many to be the upper limit of normal. In patients with no evidence of cardiac dysfunction, a QTc of > 440 ms can be associated with a 2.3 times higher risk for sudden death compared with a QTc of < 440 ms.
The most common type of acquired QTc interval prolongation is caused by drugs. Torsades de pointes occur in 2% to 8% of patients receiving quinidine and in 2% to 4% of patients receiving sotalol (Betapace®). However, it is well recognized that non-cardiac medications, including certain anti-infectives, antidepressants, anti-histamines, anti-psychotics, antineoplastics, and gastro-intestinal agents, also can adversely affect ECG parameters. In some cases, these effects have led to withdrawals of drugs from the market, or significant changes in their use. For instance, cisapride (Propulsid®) can increase the QT interval, particularly when prescribed in combination with medications that inhibit its hepatic metabolism by the cytochrome (CYP) 450 3A4 isoenzyme. Because of the risk of cardiac arrhythmias, the use of cisapride has been voluntarily withdrawn from the market in July 2000; however, there is a restricted access program.
The antihistamines, terfenadine (Seldane®) and astemizole (Hismanal®), were removed from the US market due to the potential for QT prolongation and cardiac arrhythmias when used concomitantly with certain medications that inhibited their metabolism. The withdrawal of these drugs, because of concerns over cardiac safety, has raised awareness of the potential for drug-induced ECG effects, including QT interval prolongation.
Effects on ECG Parameters
Ondansetron, granisetron, and dolasetron have all been associated with minor cardiovascular and ECG changes. Clinically relevant cardiovascular effects have not been reported to date; however, there have been rare reports of hypotension, angina, tachycardia, bradycardia, atrial fibrillation, syncope, and small, transient, reversible changes in ECG parameters with all of the available 5-HT3 receptor antagonists. Alterations in ECG intervals appear to be a class effect. Specific data about cardiovascular changes associated with this class of drugs are limited; the most detailed information that is available relates to dolasetron. As dolasetron was the last of the three agents in clinical development, most of the trials involving dolasetron included specific ECG monitoring, resulting in more detailed ECG data for this compound.
In models
Sodium channels were blocked in a frequency dependent manner by each of the 5-HT3 receptor antagonists, with granisetron having the most potent effect followed by dolasetron and ondansetron.Ondansetron displayed the most potent blockade of the rapid-delayed rectifier potassium ion channel, followed by granisetron, then dolasetron. None of the drugs displayed high-affinity block (ie, minimal effect) of the slow-delayed rectifier potassium channel. Specifically, dolasetron caused acute effects on the ECG via sodium channel blockade to delay ventricular depolarization, manifested as prolonged PR and QRS duration, without a substantial effect on ventricular repolarization (QT interval). Ondansetron primarily blocks potassium channels, prolonging the QT interval. Granisetron blocks both sodium and potassium channels, potentially affecting both depolarization and repolarization through its prolongation of PR, QRS, and QT intervals. These data help to clarify the molecular mechanisms by which some of the ECG changes (particularly QT and QRS prolongation) associated with this class of agents occur.
Clinical Trials
The propensity of these agents to prolong the QT interval has been studied in clinical trials in healthy volunteers, in cancer patients receiving chemotherapy and in surgical patients (See Table 2 and Table 3).
As a class, they may induce some statistically significant changes in QT intervals, but these changes are acute, transient, reversible, and not clinically relevant. In clinical trials to date, ECG interval increases have not resulted in clinically relevant cardiovascular events. It is important to note, however, that these clinical trials have generally excluded the participation of patients with cardiovascular disease. Computer-generated ECG analysis was used in the majority of trials because it more accurately determines ECG intervals than visual inspection of ECG tracings.
Table 2. Select Clinical Trials in Healthy Volunteers
| Study | N | Antiemetic treatments | Electrocardiogram assessments | Electrocardiographic effects |
|---|---|---|---|---|
| Hunt 1995 Randomized, double-blind, placebo-controlled dose-ranging (single-dose) |
80 | D:0.6-, 0.8-, 1-,
1.25-, 1.5-, 1.75-, 2-, 2.25-, 2.5-, 2.75-, 3-, 3.5-, 4-; 4.5-, and 5-mg/kg IV over 10 min Placebo |
Prior
to antiemetic therapy (baseline), at 1-2 h after antiemetic, and at end
of evaluation period (48 or 72 h) |
1. Reversible changes
in PR and QRS complex durations. 2. Patients were asymptomatic. 3. None of the QTc interval measurements were > 420 msec, even at the highest dose. |
| Benedict
1996 Randomized, placebo-controlled, single-blind, 5-way crossover |
30 | D:1.2-, 1.8-, or 2.4-mg/kg
IV over 15 min O: 32 mg IV over 15 minutes Placebo |
Prior to antiemetic therapy (baseline), and at 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after antiemetic | 1. Acute, transient,
and asymptomatic ECG interval changes with D and O. 2. None of the QTc interval measurements were >420 msec, even at the highest dose. 3. Increases in QT and QTc intervals were statistically significant, but not considered clinically significant 4. All changes returned to baseline by 8 h. |
| Boike
1997 Randomized, double-blind, double-dummy, 4-period, crossover |
13 | G:
10 mcg/kg IV over either 30 sec or 5 min O: 32 mg IV Placebo |
Prior to antiemetic therapy, after infusion, and at intervals up to 24 hours after the third injection | 1. O,G, and placebo
all prolonged QTc interval without demonstrable clinical effects, although
QTc interval was significantly prolonged with O versus G or placebo. 2. No significant differences between O, G, or placebo in other ECG intervals, although all were slightly prolonged. 3. No prolongation of any ECG interval was clinically meaningful. |
| Upward
1990 Single-blind, placebo- controlled, dose-escalation crossover |
8 | G:
2.5-300 mcg/kg IV over 30 min G: 40-160 mcg/kg IV over 3 min Placebo |
Prior to antiemetic therapy (baseline) and at end of infusion | 1. Only results for
the 300 mcg/kg dose were reported. 2. No clinically significant changes in pulse, blood pressure, or ECG intervals during or after antiemetic admininstration. |
Table 3. Select Trials in Patients Receiving Chemotherapy
| Study | N | Antiemetic treatments Chemotherapy | Electrocardiogram assessments | Electrocardiographic effects |
|---|---|---|---|---|
| Lifsey 1993 NR (abstract) |
20 | D: 1.8-3 mg/kg IV O: 32 mg IV Cisplatin |
Prior to antiemetic therapy and chemotherapy, and repeated 1-2 h after antiemetic | 1. Small QTc, PR,
and QRS prolongations. 2. No cardiovascular events were associated with these ECG changes |
| Baltzer
1994 NR (abstract) |
45 | D: 2.4 mg/kg IV O: 32 mg IV Cisplatin |
Prior to antiemetic therapy and at 2 h and 24 h after injection | 1. Minor, asymptomatic,
reversible prolongations in PR, QRS, QT and QTc intervals that resolved
by 24 h. 2. Mean changes at 2 h post dose significantly (P < .05) different from baseline for PR, QRS, and QT for both agents. |
| Wantanabe
1995 Open-label, observational |
12 | G:
50 mcg/kg IV over 10 min Methrotrexate, vincristine, cisplatin, pirarubicin, bleomycin, ifosfamide, or actinomycin D |
Continuous ECG monitoring 1 h prior to antiemetic therapy and continuing for 5 days | 1. In 4 patients:
ECG changes: bradycardia, P-wave changes, junctional escape beats, and
atrioventricular block.
2. All ECG changes transient and returned to baseline. 3. All patients exhibited some degree of bradycardia, but HR suppression not statistically significant versus baseline. |
| Jantunen
1996 Open-label, observational |
30 | G:
3 mg IV over 5 min Doxorubicin or epirubicin |
Prior antiemetic dose, immediately after end of granisetron, and following chemotherapy administration | No clinically significant changes in PR interval, QRS duration, or QTc interval. |
| Hesketh 1996 Randomized, double-blind, parallel |
609 | D:
1.8 or 2.4 mg/kg IV O: 32 mg IV Cisplatin |
Before antiemetic and repeated at 1-2 h and at 24 h | 1. Small, transient,
and clinically insignificant changes in PR, QRS, QT, QTc, and JT intervals
for all regimens versus baseline.
2. No increased risk of cardiovascular events with ECG changes. |
| Lofters
1997 Multicenter, randomized, 6-parallel arm |
696 | D:
2.4 mg/kg IV single dose + 200 mg oral x 6 days O: 32 mg IV or 8 mg oral twice daily x 6 days Placebo Doxorubicin, epirubicin, cisplatin, carboplatin, and/or cyclophosphamide |
Prior to antiemetic therapy and at 1-2 h and 8 days | 1. ECG changes with
both agents, but more frequent with D versus O.
2. QTc and QRS interval prolongations the most common ECG changes, but did not result in clinical events or symptoms. |
| Audhuy
1997 Multicenter, randomized, double-blind, double-dummy, 3-arm |
474 | D:
1.8 or 2.4 mg/kg IV G: 3 mg IV Cisplatin |
Prior to (<3 days) antiemetic therapy, and at 1-2 h and at 24 h after antiemetic | 1. Small increases
in PR, QRS, and QTc intervals with all regimens, but not clinically significant.
2. At 1-2 h postdose,
QTc and PR intervals statistically significantly longer (P=.0016 and P=
.0002, respectively) with D versus G, but no differences between treatments
at 24 h. No baseline specifics reported. |
D: Dolasetron; G: Granisteron; O: Ondansetron
Summary
Agents with the potential to prolong QT intervals, characterized by prolonged ventricular repolarization with QT intervals exceeding 500 ms, pose a serious and clinically relevant complication in general practice, as they may ultimately give rise to a potentially fatal arrhythmia, torsades de pointes. The 5-HT3 receptor antagonists have been associated with QT interval prolongation, but have a low proarrhythmic risk which is not associated with the occurrence of potentially lethal arrhythmias. To date, no cases of torsades de pointes associated with 5-HT3 receptor antagonist administration have been reported in the medical literature. Typically, the QT interval changes with the 5-HT3 receptor antagonists do not exceed 15 milliseconds.
Both the American Society of Health-System Pharmacists (ASHP) Therapeutic Guidelines on the Pharmacologic Management of Nausea and Vomiting in Adult and Pediatric Patients Receiving Chemotherapy or Radiation Therapy or Undergoing Surgery and the Recommendations for the Use of Antiemetics published by the American Society of Clinical Oncology (ASCO) state that there are no differences in the safety profiles of the available 5-HT3 receptor antagonists, including ECG effects.
Conclusions
The 5-HT3 receptor antagonists are effective in the prevention and treatment of nausea and vomiting associated with chemotherapy, radiation therapy, and surgery. Dolasetron, ondansetron, and granisetron also have, as a class, some effects on ECG intervals. Reversible, transient changes in the PR, QRS, and QT intervals have been consistently observed in noncomparative and comparative trials. Ondansetron, granisetron, and dolasetron all have a similar propensity to affect ECG intervals; however, clinically relevant ECG changes have not been reported or documented.
References available upon request