Cleveland Clinic Basic and Clinical Immunology for the Busy Clinician Online Series
COVID-19: A Tocsin to our Aging, Unfit, Corpulent, and Immunodeficient Society
David C. Nieman, DrPH, FACSM
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19) spread quickly to all parts of Earth during 2020 causing widespread morbidity and mortality. Intense scientific scrutiny during the first year of the COVID-19 pandemic has greatly broadened our understanding of SARS-CoV-2. This virus is highly transmissible through airborne droplets, with 40-45% of infections occurring in asymptomatic individuals that account for at least half of all known new cases (1,2). Although 80% of COVID-19 cases result in mild-to-moderate symptomatology, one in five develop significant symptoms. COVID-19 symptoms can persist for at least 6 months after acute infection, with fatigue, muscle weakness, sleep difficulties, anxiety and depression, and reductions in 6-minute walking test performance commonly reported by post-acute sequelae of SARS-CoV-2 infection (PASC), or “long COVID”, survivors (3,4). A considerable proportion (22-56% depending on disease severity) have pulmonary diffusion abnormalities at least 6 months after symptom onset (3).
This review will emphasize that physical activity has potential value at each of the three prevention levels for COVID-19 (Figure 1). At the primary prevention level, several new epidemiological studies support that physical activity lowers the risk for COVID-19 (see Table 1). These data add to the growing awareness of physical activity’s role in improving immunosurveillance against pathogens and in lowering risk for a variety of respiratory infections. Secondary prevention strategies include vaccination. Although existing data are limited, some studies support that the immune response to vaccination is augmented in lean and fit individuals. At the tertiary prevention level, exercise rehabilitation has the potential to help PASC patients counter symptoms of fatigue and muscle weakness, similar to what has been reported for patients with chronic fatigue syndrome or those recovering from cancer treatment.
Primary Prevention: Physical Activity, Immunity, and COVID-19 Risk
Mounting evidence supports a protective role of regular physical activity against a variety of respiratory infections (i.e., risk, incidence, severity, and mortality) (5-7). As summarized in Figure 2, risk for bacterial and viral infectious disease mortality was more than 50% lower as determined in a cohort of 97,844 adults followed for over nine years (8). Whether this protection extends to COVID-19 is currently an active area of scientific endeavor. Acute and chronic exercise has profound effects on immunosurveillance, as reviewed recently elsewhere (9,10).
Demographic and lifestyle risk factors for severe illness from COVID-19 include older age, male sex, obesity (in all age groups), cigarette smoking, and underlying medical conditions (many of which are related to obesity) (11-14). Although physical inactivity has not yet been recognized by health agencies as a COVID-19 risk factors, mounting epidemiological evidence indicates an independent influence.
As summarized in Table 1, several studies have been published using data from the UK Biobank (UKBB) prospective cohort. In one UKBB analysis, physical inactivity was related to a 32% increased risk for COVID-19 hospitalizations in 387,109 adults (8). Cigarette smoking (42% added risk) and obesity (105%) when combined with physical inactivity increased the risk of COVID-19 hospitalization 4.4-fold compared to optimal lifestyles. Unhealthy behaviors in combination accounted for up to 51% of the population attributable fraction of severe COVID-19 (8). In another UKBB analysis, higher levels of physical activity as measured with accelerometers was linked to a 20-26% reduced chance of being diagnosed with COVID-19 (15). In this same cohort, a poor balance between physical activity and sleep/rest was related to a 29% increased risk for severe COVID-19 (16). An additional UKBB analysis showed that low physical fitness (as measured by a slow walking pace) was strongly related to confirmed COVID-19 infection, and that obesity was a unifying risk factor (6). A retrospective observational study within the Henry Ford Medical Group showed an independent and inverse association between maximal exercise capacity and likelihood of COVID-19 hospitalization (17). In this study, the odds for COVID-19 hospitalization were 13% lower for every one MET higher level for exercise capacity measured during graded exercise tests.
These are important data supporting the public health strategy of facilitating physical activity during the COVID-19 and future pandemics (18). Public health officials will debate the overall health impact of COVID-19 related social distancing measures for years to come. An analysis of 455,404 individuals from 187 countries showed that within 30 days of the COVID-19 pandemic declaration by the World Health Organization, mean step counts decreased 27.3% (19). This decrease in physical activity came during a time when regular physical activity was most needed. Physical activity has clear linkages to immune function and overall physical and mental health. Prolonged social distancing (at least not without guidance on how to maintain or increase physical activity) may not be the best public health strategy as we continue to battle current and future viral pandemics (12).
Regular physical activity improves immunosurveillance against pathogens and reduces morbidity and mortality from viral infection and acute respiratory illness (9,10,12). Each 30-to-60-minute moderate-to-vigorous exercise bout improves overall surveillance against pathogens by stimulating the ongoing exchange of important types of white blood cells between the circulation and tissues. These immune cells that are activated and recruited during acute exercise bouts include neutrophils, macrophages, natural killer cells, cytotoxic T cells, and immature B cells. Over time, regular exercise-induced increases in antipathogenic leukocytes reduce infectious disease illness risk, and lower systemic inflammation. Thus, regular exercise training can be viewed as an immune system adjuvant and is of particular clinical value for obese individuals with comorbidities and older individuals. In a COVID-19 “call to action statement”, the American College of Sports Medicine (ACSM) recommended that individuals maintain immune health by participating in moderate physical activity 150-300 minutes per week and keeping body weight at normal levels (10).
The immune system is broadly divided into the innate and adaptive arms, and have separate and linked tasks (20-22). The innate immune system functions as the first line of host defense against microbial infections (22). Innate immune signaling and inflammasome activation are well-established key barriers during viral infection. However, activation of the innate immune system must be tightly regulated, because excessive activation can lead to systemic inflammation and tissue damage. SARS-CoV-2 is unusually effective at evading the triggering of early innate immune responses by blocking type 1 interferons (IFNs), and this allows the virus to multiply quickly (20). The adaptive immune system is important for control of most viral infections including SARS-CoV-2 (20). The adaptive immune system consists of three major cell types: B cells, CD4+ T cells, and CD8+ T cells. B cells produce antibodies, CD8+ T cells excel in killing infected cells, and CD4+ T cells have helper and effector functions.
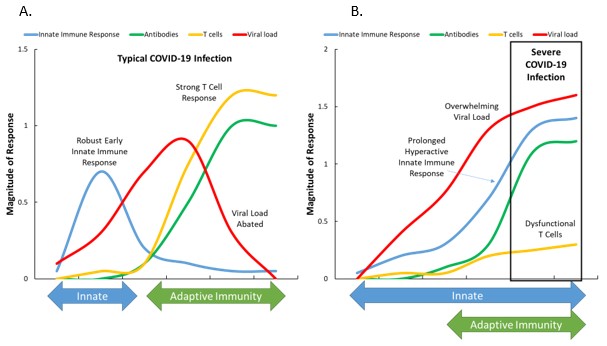
The immune response to SARS-CoV-2 is summarized in Figure 3 for typical and severe COVID-19 cases (20). The magnitude of the antibody response against SARS-CoV-2 is highly heterogeneous between individuals (20). In a typical COVID-19 infection, SARS-CoV-2 infects cells by attaching to the viral entry receptor, ACE2, and is then recognized by pattern recognition receptors (23). In response, the innate immune system is activated, but the unique interferon (IFN)-blocking ability of SARS-CoV-2 (more so in men than women) allows the virus to multiply (21,23). Low IFN production from the innate immune system has been strongly associated with high SARS-CoV-2 viral burden and COVID-19 severity (20). The adaptive immune system’s T and B cells respond slowly (6-10 days) but vigorously, with an increase in SARS-CoV-2 specific antibodies, and ultimately a decrease in the viral load (20). Most SARS-CoV-2 infected individuals (90%) seroconvert by day 10 post-symptom onset (20).
T cells, however, are dysfunctional in older and obese individuals with related comorbidities, and exhibit a dysregulated status of activation and function (21,23). Early in life, nearly all T lymphocytes express CD45RA, a typical marker of naïve cells that are more capable of responding to a novel pathogen such as SARS-CoV-2 (21). In contrast, older individuals have a higher proportion of memory versus naïve T cells, impairing the immune system’s capacity to clear SARS-CoV-2 (21).
The adaptive immune response in these individuals is inadequate to stop the virus, and is compensated in part by an overactive innate immune response (20). As a result, the patient experiences excessive inflammation, tissue damage, high viral loads, and severe COVID-19 (20) (Figure 3). Immune changes linked to severe cases of COVID-19 include (20-26): 1) an increased ratio of neutrophils to lymphocytes (Ne/Ly); 2) elevated cytokines [interleukin (IL)-1β, IL-2R, IL-6, IL-8, IL-10, IL-15, IL-18, granulocyte-colony stimulating factor (GCSF), monocyte chemoattractant protein (MCP)-1, and tumor necrosis factor (TNF)-α]; 3) T cell dysregulation; 4) decreased lymphocyte counts for regulatory T cells (Tregs), CD4+ T cells, and CD8+ T cells; 5) a low lymphocyte-to-CRP ratio (LCR); and NLR family pyrin domain containing 3 (NLRP3) inflammasome activation (21). Most of these immune measures with severe COVID-19 are very similar in magnitude to those measured transiently in endurance athletes during recovery from competitive events (9). In the rested and uninfected state, lean and fit individuals have very low levels of systemic inflammation, low Ne/Ly and LCR, and strong innate and adaptive immunity responses, even in older age (9). The implication is that the immune systems in physically active, nonobese adults are less "primed" to respond in a negative fashion following SARS-CoV-2 infection.
Much more remains to be learned about SARS-CoV-2, including issues related to pre-existing immunity from other coronaviruses, why immune responses and disease severity vary so widely between individuals, the potential for reinfection, vaccine elicited immunity and protection, how durable protection is over the long term, potency of variants, and how to improve immune memory (20).
Secondary Prevention: Physical Activity and COVID-19 Vaccination
Building on years of basic science, several vaccines with high efficacy were developed within a year of the COVID-19 pandemic. The duration of immune memory and protective immunity in response to COVID-19 vaccines for varying demographic and lifestyle groups will be an active area of research for years to come.
Obesity impairs the efficacy of the influenza vaccine, and the early concern was that the same finding would apply to COVID-19 vaccines. Initial results from the Pfizer-BioNTech and Moderna COVID-19 vaccine trials, however, indicated similar efficacy among individuals with and without obesity (27). Additional studies will provide data on long-term vaccine efficacy, particularly in high-risk groups.
Although not conclusive, several studies indicate that acute or chronic exercise augments the antibody titer response to influenza vaccinations (28-33). Whether these data apply to COVID-19 vaccines remains to be determined. The most impressive data support that long-term exercise training improves the immune response to influenza vaccination. Four studies support that moderate exercise training by older adults enhances the mean fold increase in antibody titer after influenza immunization (30-33). One cross-sectional study, for example, of older adults showed that those exercising vigorously for 20 minutes per session three or more time per week had higher levels of influenza specific IgG and IgM compared to sedentary peers (33). T cell function as measured with peripheral blood mononuclear cell (PBMC) proliferation was highest in the vigorous exercise group, similar to findings from other studies (34).
Influenza vaccination in athletes has been found to be safe and effective whether administered 2 hours or 24-26 hours after a training bout (35). One study showed that the increase in influenza virus specific T-cells and neutralizing antibodies was more pronounced in elite athletes compared to controls (36).
Taken together, the data suggest that vaccine efficacy is positively related to a higher level of physical fitness through regular exercise training. Whether this applies to highly effective mRNA-based COVID-19 vaccines remains to be proven.
Physical Activity and Rehabilitation from COVID-19
Ongoing studies indicate that morbidity of COVID-19 illness is prolonged with a significant symptom burden for many patients. The case definition for PASC has not yet been defined but is typically understood to be a collection of symptoms following COVID-19 that persist for more than 28 days (3,4). A survey of 3,762 respondents from 56 countries showed that the most common systemic symptoms were fatigue, post-exertional malaise (PEM), elevated temperature, chills/flushing/sweats, skin sensations, and weakness. Other common specific symptoms came from multiple organ systems and included sore throat, blurred vision, shortness of breath and dry cough, heart palpitations, tightness of chest, muscle aches, diarrhea, anxiety, irritability, depression, memory loss, headaches, balance issues, brain fog, sleep difficulties, and poor attention (4). Patients rated fatigue, breathing issues, and cognitive dysfunction as the most debilitating symptoms. Relapses were common and occurred in an irregular pattern, with exercise and mental stress as the main triggers. About two-thirds of respondents experienced symptoms for at least 6 months.
Many of these symptoms are similar to myalgic encephalomyelitis, chronic fatigue syndrome (ME/CFS) and postural orthostatic tachycardia syndrome (POTS) (37-39). PEM is one of the three required symptoms for ME/CFS, along with unrefreshing sleep and a reduction in ability to engage in pre-illness levels of activity. Of the respondents who experienced PEM (89%), about half experienced it immediately after exercise or the same day, and the other half the following 1 to 3 days (38).
ME/CFS is a debilitating disorder that affects up to 2.5 million Americans. Infections from viruses, and immunologic and endocrine disorders may be underlying causes for ME/CFS (38,39). No effective treatment has been approved for patients with ME/CFS, but cognitive behavior therapy and graded exercise therapy are typically recommended as effective therapies for ME/CFS (37). Physical activity with a gradual increase in intensity is recommended for ME/CFS and long COVID, but with care to avoid a worsening of symptoms.
Evidence-based recommendations for return-to-exercise guidelines are limited, but rest and no exercise for two weeks from a positive COVID-19 test result is encouraged under the guidance of a health care team. A slow resumption of exercise training is then recommended with close monitoring for clinical deterioration. This is a conservative approach until more is known about the interaction between SARS-CoV-2, heavy exertion, and heart health (10,12,40).
A critical concern for highly active individuals and competitive athletes is the potential for cardiac injury from SARS-CoV-2 (41,42). Acute cardiac injury and myocarditis have been observed in a significant proportion of hospitalized patients with COVID-19, and exercise could accelerate viral replication and cardiac damage. There is increasing concern that athletes may be susceptible to COVID-19-induced myocarditis. Emerging data support COVID-19-induced cardiac injury in non-athletes in the general community. At present, there are insufficient data to support cardiac magnetic resonance imaging (CMR) of all athletes. CMR is appropriate when clinical symptoms and objective pathologic criteria are suggestive of myocarditis (41).
Conclusions
This article emphasized that physical activity has potential benefits to counter COVID-19 infection at three prevention levels. Several epidemiological studies support that regular physical activity is associated with a reduced risk for COVID-19. Although specific COVID-19 related studies are needed, data from studies with other types of infectious agents support the potential role for physical activity to augment COVID-19 vaccine efficacy and to improve the quality of life for PACS patients.
Annotated Bibliography
- Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review. Ann Intern Med. 2020 Sep 1;173(5):362-367. PMID: 32491919.
- Johansson MA, Quandelacy TM, Kada S, Prasad PV, Steele M, Brooks JT, Slayton RB, Biggerstaff M, Butler JC. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw Open. 2021 Jan 4;4(1):e2035057. PMID: 33410879.
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021 Jan 16;397(10270):220-232. PMID: 33428867.
- Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, Redfield S, Austin JP, Akrami A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. medRxiv 2020.12.24.20248802; doi: https://doi.org/10.1101/2020.12.24.20248802.
- Song Y, Ren F, Sun D, Wang M, Baker JS, István B, Gu Y. Benefits of Exercise on Influenza or Pneumonia in Older Adults: A Systematic Review. Int J Environ Res Public Health. 2020 Apr 13;17(8):2655. PMID: 32294922.
- Ho FK, Celis-Morales CA, Gray SR, Katikireddi SV, Niedzwiedz CL, Hastie C, Ferguson LD, Berry C, Mackay DF, Gill JM, Pell JP, Sattar N, Welsh P. Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. BMJ Open. 2020 Nov 19;10(11):e040402. PMID: 33444201.
- Min C, Yoo DM, Wee JH, Lee HJ, Byun SH, Choi HG. Mortality and cause of death in physical activity and insufficient physical activity participants: a longitudinal follow-up study using a national health screening cohort. BMC Public Health. 2020 Sep 29;20(1):1469. PMID: 32993602.
- Hamer M, O'Donovan G, Stamatakis E. Lifestyle risk factors, obesity and infectious disease mortality in the general population: Linkage study of 97,844 adults from England and Scotland. Prev Med. 2019 Jun;123:65-70. PMID: 30844499.
- Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019 May;8(3):201-217. PMID: 31193280.
- Denay KL, Breslow RG, Turner MN, Nieman DC, Roberts WO, Best TM. ACSM Call to Action Statement: COVID-19 Considerations for Sports and Physical Activity. Curr Sports Med Rep. 2020 Aug;19(8):326-328. PMID: 32769667.
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020 Aug;584(7821):430-436. PMID: 32640463.
- Nieman DC. Coronavirus disease-2019: A tocsin to our aging, unfit, corpulent, and immunodeficient society. J Sport Health Sci. 2020 Jul;9(4):293-301. PMID: 32389882.
- Ioannou GN, Locke E, Green P, Berry K, O'Hare AM, Shah JA, Crothers K, Eastment MC, Dominitz JA, Fan VS. Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10 131 US Veterans With SARS-CoV-2 Infection. JAMA Netw Open. 2020 Sep 1;3(9):e2022310. PMID: 32965502.
- Huang Y, Lu Y, Huang YM, Wang M, Ling W, Sui Y, Zhao HL. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020 Dec;113:154378. PMID: 33002478.
- Zhang X, Li X, Sun Z, He Y, Xu W, Campbell H, Dunlop MG, Timofeeva M, Theodoratou E. Physical activity and COVID-19: an observational and Mendelian randomisation study. J Glob Health. 2020 Dec;10(2):020514. PMID: 33312507.
- Rowlands AV, Kloecker DE, Chudasama Y, Davies MJ, Dawkins NP, Edwardson CL, Gillies C, Khunti K, Razieh C, Islam N, Zaccardi F, Yates T. Association of Timing and Balance of Physical Activity and Rest/Sleep With Risk of COVID-19: A UK Biobank Study. Mayo Clin Proc. 2021 Jan;96(1):156-164. PMID: 33413813.
- Brawner CA, Ehrman JK, Bole S, Kerrigan DJ, Parikh SS, Lewis BK, Gindi RM, Keteyian C, Abdul-Nour K, Keteyian SJ. Inverse Relationship of Maximal Exercise Capacity to Hospitalization Secondary to Coronavirus Disease 2019. Mayo Clin Proc. 2021 Jan;96(1):32-39. PMID: 33413833.
- Lange KW, Nakamura Y. Lifestyle factors in the prevention of COVID-19. Glob Health J. 2020 Dec;4(4):146-152. PMID: 33520339.
- Tison GH, Avram R, Kuhar P, Abreau S, Marcus GM, Pletcher MJ, Olgin JE. Worldwide Effect of COVID-19 on Physical Activity: A Descriptive Study. Ann Intern Med. 2020 Nov 3;173(9):767-770. PMID: 32598162.
- Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021 Jan 12:S0092-8674(21)00007-6. PMID: 33497610.
- de Candia P, Prattichizzo F, Garavelli S, Matarese G. T Cells: Warriors of SARS-CoV-2 Infection. Trends Immunol. 2021 Jan;42(1):18-30. PMID: 33277181.
- Lee S, Channappanavar R, Kanneganti TD. Coronaviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines. Trends Immunol. 2020 Dec;41(12):1083-1099. PMID: 33153908.
- Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021 Jan;27(1):28-33. PMID: 33442016.
- Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020 May 1;130(5):2620-2629. PMID: 32217835.
- Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, Hu JL, Xu W, Zhang Y, Lv FJ, Su K, Zhang F, Gong J, Wu B, Liu XM, Li JJ, Qiu JF, Chen J, Huang AL. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020 Aug;26(8):1200-1204. PMID: 32555424.
- Garcia-Beltran WF, Lam EC, Astudillo MG, Yang D, Miller TE, Feldman J, Hauser BM, Caradonna TM, Clayton KL, Nitido AD, Murali MR, Alter G, Charles RC, Dighe A, Branda JA, Lennerz JK, Lingwood D, Schmidt AG, Iafrate AJ, Balazs AB. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021 Jan 21;184(2):476-488.e11. PMID: 33412089.
- Townsend MJ, Kyle TK, Stanford FC. COVID-19 Vaccination and Obesity: Optimism and Challenges. Obesity (Silver Spring). 2021 Jan 28. PMID: 33506642.
- Edwards KM, Burns VE, Reynolds T, Carroll D, Drayson M, Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav Immun. 2006 Mar;20(2):159-68. PMID: 16102936.
- Edwards KM, Burns VE, Allen LM, McPhee JS, Bosch JA, Carroll D, Drayson M, Ring C. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav Immun. 2007 Feb;21(2):209-17. PMID: 16824730.
- Monteiro FR, Roseira T, Amaral JB, Paixão V, Almeida EB, Foster R, Sperandio A, Rossi M, Amirato GR, Apostólico JS, Santos CAF, Felismino ES, Leal FB, Thomazelli LM, Durigon EL, Oliveira DBL, Vieira RP, Santos JMB, Bachi ALL. Combined Exercise Training and l-Glutamine Supplementation Enhances Both Humoral and Cellular Immune Responses after Influenza Virus Vaccination in Elderly Subjects. Vaccines (Basel). 2020 Nov 16;8(4):685. PMID: 33207604.
- de Araújo AL, Silva LC, Fernandes JR, Matias Mde S, Boas LS, Machado CM, Garcez-Leme LE, Benard G. Elderly men with moderate and intense training lifestyle present sustained higher antibody responses to influenza vaccine. Age (Dordr). 2015 Dec;37(6):105. PMID: 26480853.
- Kohut ML, Arntson BA, Lee W, Rozeboom K, Yoon KJ, Cunnick JE, McElhaney J. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine. 2004 Jun 2;22(17-18):2298-306. PMID: 15149789.
- Kohut ML, Cooper MM, Nickolaus MS, Russell DR, Cunnick JE. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci. 2002 Sep;57(9):M557-62. PMID: 12196490.
- Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE, Nehlsen-Cannarella SL. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993 Jul;25(7):823-31. PMID: 8350705.
- Stenger T, Ledo A, Ziller C, Schub D, Schmidt T, Enders M, GÄrtner BC, Sester M, Meyer T. Timing of Vaccination after Training: Immune Response and Side Effects in Athletes. Med Sci Sports Exerc. 2020 Jul;52(7):1603-1609. PMID: 31977634.
- Ledo A, Schub D, Ziller C, Enders M, Stenger T, Gärtner BC, Schmidt T, Meyer T, Sester M. Elite athletes on regular training show more pronounced induction of vaccine-specific T-cells and antibodies after tetravalent influenza vaccination than controls. Brain Behav Immun. 2020 Jan;83:135-145. PMID: 31580932.
- Kim DY, Lee JS, Park SY, Kim SJ, Son CG. Systematic review of randomized controlled trials for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. 2020 Jan 6;18(1):7. PMID: 31906979.
- Joseph P, Arevalo C, Oliveira RKF, Faria-Urbina M, Felsenstein D, Oaklander AL, Systrom DM. Insights from Invasive Cardiopulmonary Exercise Testing of Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Chest. 2021 Feb 9:S0012-3692(21)00256-7. PMID: 33577778.
- Nacul L, O'Boyle S, Palla L, Nacul FE, Mudie K, Kingdon CC, Cliff JM, Clark TG, Dockrell HM, Lacerda EM. How Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Progresses: The Natural History of ME/CFS. Front Neurol. 2020 Aug 11;11:826. PMID: 32849252.
- Phelan D, Kim JH, Chung EH. A Game Plan for the Resumption of Sport and Exercise After Coronavirus Disease 2019 (COVID-19) Infection. JAMA Cardiol. 2020 May 13. PMID: 32402054.
- Kim JH, Levine BD, Phelan D, Emery MS, Martinez MW, Chung EH, Thompson PD, Baggish AL. Coronavirus Disease 2019 and the Athletic Heart: Emerging Perspectives on Pathology, Risks, and Return to Play. JAMA Cardiol. 2021 Feb 1;6(2):219-227. PMID: 33104154.
- Phelan D, Kim JH, Elliott MD, Wasfy MM, Cremer P, Johri AM, Emery MS, Sengupta PP, Sharma S, Martinez MW, La Gerche A. Screening of Potential Cardiac Involvement in Competitive Athletes Recovering From COVID-19: An Expert Consensus Statement. JACC Cardiovasc Imaging. 2020 Dec;13(12):2635-2652. PMID: 33303102.
Asymptomatic persons seem to account for approximately 40% to 45% of SARS-CoV-2 infections, and they can transmit the virus to others for an extended period, perhaps longer than 14 days.
Transmission from asymptomatic individuals was estimated to account for more than half of all transmissions. In addition to identification and isolation of persons with symptomatic COVID-19, effective control of spread will require reducing the risk of transmission from people with infection who do not have symptoms.
At 6 months after acute infection, COVID-19 survivors were mainly troubled with fatigue or muscle weakness, sleep difficulties, and anxiety or depression. Patients who were more severely ill during their hospital stay had more severe impaired pulmonary diffusion capacities and abnormal chest imaging manifestations, and are the main target population for intervention of long-term recovery.
Patients with Long COVID report prolonged multisystem involvement and significant disability. Most had not returned to previous levels of work by 6 months.
Most of the current studies suggested that prolonged moderate aerobic exercise may help to reduce the risk of influenza-related infection and improve the immune responses to influenza or pneumonia vaccination in older adults.
Modification of lifestyle may help to reduce the risk of COVID-19 and could be a useful adjunct to other interventions, such as social distancing and shielding of high risk.
Physical activity is inversely associated with mortality from mental, respiratory, cancer, and cardiovascular conditions. Results were consistent across age and obesity subgroups.
Compared to physically inactive participants both insufficiently active and sufficiently active (at least 150 min/wk. moderate - vigorous activity) was associated with reduced risk of infectious disease mortality in models mutually adjusted for other lifestyle factors.
This review summarizes research discoveries within 4 areas of exercise immunology that have received the most attention from investigators: (1) acute and chronic effects of exercise on the immune system, (2) clinical benefits of the exercise-immune relationship, (3) nutritional influences on the immune response to exercise, and (4) the effect of exercise on immunosenescence.
This call to action encourages individuals to start or continue moderate physical activity for 150 to 300 minutes per week. Additionally, evaluate recovered COVID-19 individuals for exercise tolerance to promote a safe return to exercise.
COVID-19-related death was associated with: being male, greater age and deprivation, diabetes, severe asthma, and various other medical conditions. Compared with people of white ethnicity, Black and South Asian people were at higher risk, even after adjustment for other factors.
Two primary prevention strategies to reduce the risk for COVID-19 at both the community and individual levels include mitigation activities and the adoption of lifestyle practices consistent with good immune health. Animal and human studies support the idea that, in contrast to high exercise workloads, regular moderate-intensity physical activity improves immunosurveillance against pathogens and reduces morbidity and mortality from viral infection and respiratory illnesses including the common cold, pneumonia, and influenza.
Most SARS-CoV-2 deaths in VA patients were associated with older age, male sex, and comorbidity burden.
Obesity increases risk for hospitalization, ICU admission, and death among patients with COVID-19. Further, excessive visceral adiposity appears to be associated with severe COVID-19 outcomes.
Results indicate a protective effect of objectively measured physical activity and COVID-19 outcomes independent of age, sex, measures of obesity, and smoking status. Policies to encourage and facilitate exercise at a population level during the pandemic should be considered.
Results highlight the importance of not just physical activity, but also quality sleep/rest and regular sleep/rest patterns, on risk of COVID-19.
Peak METs were inversely associated with the likelihood of hospitalization in unadjusted (odds ratio, 0.83) and adjusted models (odds ratio, 0.87). Maximal exercise capacity is independently and inversely associated with the likelihood of hospitalization due to COVID-19.
Appropriate lifestyle changes in regard to nutrition, exercise, sleep, smoking and alcohol intake may help shift the population distribution of infection risk and aid in preventing severe COVID-19 disease.
Worldwide, within 10 days of the pandemic declaration, there was a 5.5% decrease in mean steps (287 steps), and within 30 days, there was a 27.3% decrease in mean steps (1432 steps). There was wide regional variation in average step count change and in the timing and rate of that change.
The adaptive immune system is important for control of most viral infections. CD4+ T cells, CD8+ T cells, and neutralizing antibodies all contribute to control of SARS-CoV-2 in both non-hospitalized and hospitalized cases of COVID-19.
Severe infection with severe acute respiratory syndrome coronavirus (SARS-CoV)-2 is characterized by massive cytokine release and T cell loss. The exaggerated host immune response, incapable of viral clearance, instead aggravates respiratory distress, as well as cardiac, and/or damage to other organs.
The innate immune system acts as the first line of defense against pathogens, including coronaviruses (CoVs). This review provides a focus on the present understanding of innate immune responses, inflammasome activation, inflammatory cell death pathways, and cytokine secretion during SARS-CoV-2 infection.
Discussion of current understanding of the immunological determinants of COVID-19 disease presentation and severity, with linkages to known immune-system differences between young and old people and between men and women, and other factors associated with different disease presentations and severity.
Compared with moderate cases, severe cases had dyspnea, lymphopenia, and higher levels of alanine aminotransferase, lactate dehydrogenase, C-reactive protein, ferritin, D-dimer, IL-2R, IL-6, IL-10, and TNF-α. Absolute numbers of T lymphocytes, CD4+ T cells, and CD8+ T cells decreased in nearly all the patients, and were markedly lower in severe cases.
These data suggest that asymptomatic individuals had a weaker immune response to SARS-CoV-2 infection. The reduction in IgG and neutralizing antibody levels in the early convalescent phase might have implications for immunity strategy and serological surveys.
Results highlight the importance of neutralizing humoral immunity on disease progression and the need to develop broadly protective interventions to prevent future coronavirus pandemics.
Initial results from the Pfizer-BioNTech and Moderna COVID-19 vaccine trials, though limited by inadequate power to compare subgroups and incomplete stratification of high-risk groups, appear to have similar efficacy among individuals with and without obesity.
Participants were randomized to 45 minutes of intermittent cycling, mental stress (arithmetic task), or control (sitting and reading). Women, but not men, had higher antibody responses after exercise or mental stress, but only for one of the influenza virus strains.
Men and women engaged in 25 minutes of arm eccentric exercise or rest, and then received the influenza vaccination 6 hours later. Blood samples taken at 6, 8 and 20 weeks post-vaccination showed enhancement of the antibody titer response with exercise compared to rest in women, but not in men.
Elderly subjects who exercised regularly were compared to peers who did not engage in regular exercise. Each group was also randomized to receive glutamine or placebo supplements for 30 days after receiving the influenza vaccination. Both regular exercise training and glutamine supplementation improved the immune response to the influenza vaccine.
This cross-sectional study of elderly men showed that the antibody response to the influenza vaccine was stronger in groups who trained on a regular basis (both moderately and vigorously).
Older adults were assigned to exercise or control groups for 10 months, with influenza vaccines administered before and after the exercise intervention. Exercisers exhibited a greater mean fold increase in antibody titer to the influenza vaccine.
This cross-sectional study of elderly adults showed that anti-influenza IgG and IgM were higher in those exercising vigorously for 20 minutes or more three or more times per week.
Cross-sectional data supported higher T cell and natural killer cell function, and lower rates for upper respiratory tract infection, in highly conditioned compared to sedentary elderly women.
Healthy, high level athletes were given the influenza vaccine within 2 or 24-26 hours after the last training session. Both groups experienced similar increases in influenza specific antibodies and CD4 T cells.
High-level athletes and controls received the influenza vaccine, and the athletes experienced a more pronounced increase in influenza-specific T cells and neutralizing antibodies.
This systematic review showed therapeutic support for CFS/ME for non-pharmacological therapies including cognitive-behavior-therapy-related treatments and graded-exercise-related therapies.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) affects tens of millions worldwide, but the causes of exertional intolerance are poorly understood. The ME/CFS label overlaps with postural orthostatic tachycardia (POTS) and fibromyalgia. , and objective evidence of small fiber neuropathy (SFN) is reported in ∼50% of POTS and fibromyalgia patients. During exercise, ME/CFS patients averaged lower right atrial pressures and peak VO2 than controls.
As in other chronic diseases, ME/CFS evolves through different stages, from asymptomatic predisposition, progressing to a prodromal stage, and then to symptomatic disease. Disease incidence depends on genetic makeup and environment factors, the exposure to singular or repeated insults, and the nature of the host response.
For athletes who remain asymptomatic and are negative for COVID-19, return to exercise training is permissible without additional testing. However, asymptomatic athletes who test positive for COVID-19 antigen (active infection) should refrain from exercise training for at least 2 weeks from the date of positive test result and follow strict isolation guidelines. If athletes remain asymptomatic, slow resumption of activity should be guided under direction from their medical professional.
Cardiac injury with attendant negative prognostic implications is common among patients hospitalized with coronavirus disease 2019 (COVID-19) infection. Whether cardiac injury, including myocarditis, also occurs with asymptomatic or mild-severity COVID-19 infection is uncertain. There is an ongoing concern about COVID-19-associated cardiac pathology among athletes because myocarditis is an important cause of sudden cardiac death during exercise.
This review seeks to evaluate the current evidence regarding COVID-19-associated cardiovascular disease and how multimodality imaging may be useful in the screening and clinical evaluation of athletes with suspected cardiovascular complications of infection.
Additional reading: Nalbandian, A., Sehgal, K., Gupta, A. et al. Post-acute COVID-19 syndrome. Nat Med (2021). https://doi.org/10.1038/s41591-021-01283-z
Table 1 Epidemiological research on the relationship between physical activity, physical fitness, and COVID-19 outcomes.
| Investigators, year published | Study population | Research and statistical design | Key findings |
|---|---|---|---|
| Zhang et al., 2020 (15) | >Prospective cohort, 500,000 adults, United Kingdom Biobank (UKBB) study; 1,746 with COVID-19 (age 68.8±9.2 y, 399 deaths (age 74.7±6.0). | Logistic regression analysis of physical activity (PA) (objectively, subjectively measured) and COVID-19 outcomes; test of causality using Mendelian randomization (MR). | Protective effect (20-26%) of objectively measured PA on COVID-19 outcomes after adjustment (age, sex, obesity, smoking). MR analyses did not support a causal association. |
| Brawner et al., (17) | 246 patients (age, 59±12 y; 42% male; 75% black race) with maximal exercise capacity test, positive for SARS-CoV-2. | Logistic regression, COVID-19 hospitalization and peak metabolic equivalents (METs), with adjustment for 13 covariates. | Peak METs lower (P<.001) among hospitalized patients (6.7±2.8) vs. not hospitalized (8.0±2.4) (odds ratio, OR, adjusted model, 0.87). |
| Hamer et al., (8) | Prospective cohort of 387,109 adults, UKBB study, ages 40-69 y. | Regression models fitted to estimate RR for associations between physical activity, obesity, smoking, and alcohol consumption (baseline questionnaires) and severe COVID-19. | RRs adjusted for age, sex and each lifestyle factor were raised for physical inactivity (1.32), smoking (1.42), and obesity (2.05), but not heavy alcohol intake (1.12). |
| Ho et al., 2020 (6) | Prospective cohort, 235,928 adults (age 49-83 y), UKBB study tested for SARS-CoV-2; 397 with confirmed COVID-19. | Poisson regression of self-reported slow walking pace (fitness), demographic and modifiable risk factors for COVID-19 (relative risk, RR). | Slow walking pace linked to COVID-19 diagnosis (RR 1.53) after multivariable adjustment; BMI (RR 1.29), smoking (RR 1.39). |
| Rowlands et al., (16) | Prospective cohort of 91,248 adults (age 49-83 y), UKBB study, 207 with confirmed COVID-19, 124 severe. | Logistic regression, severe COVID-19 with physical activity (accelerometer data) and sleep/rest variables, with adjustment for age, sex, ethnicity, diet, smoking, and other factors. | Higher daytime activity related to lower risk for severe COVID-19 (OR=0.75). Higher movement during sleep/rest linked to higher risk (OR=1.26). Proper balance of activity and sleep/rest linked to a 30% lower risk of severe COVID-19. |
| Zhang et al., 2020 (15) | >Prospective cohort, 500,000 adults, United Kingdom Biobank (UKBB) study; 1,746 with COVID-19 (age 68.8±9.2 y, 399 deaths (age 74.7±6.0). | Logistic regression analysis of physical activity (PA) (objectively, subjectively measured) and COVID-19 outcomes; test of causality using Mendelian randomization (MR). | Protective effect (20-26%) of objectively measured PA on COVID-19 outcomes after adjustment (age, sex, obesity, smoking). MR analyses did not support a causal association. |
Figure 1. Physical activity has a role in combating COVID-19 morbidity and mortality at each prevention level. Regular physical activity lowers the risk for severe COVID-19, may augment the antibody response to COVID-19 vaccinations, and is projected to improve rehabilitation for PACS (long COVID-19) patients.
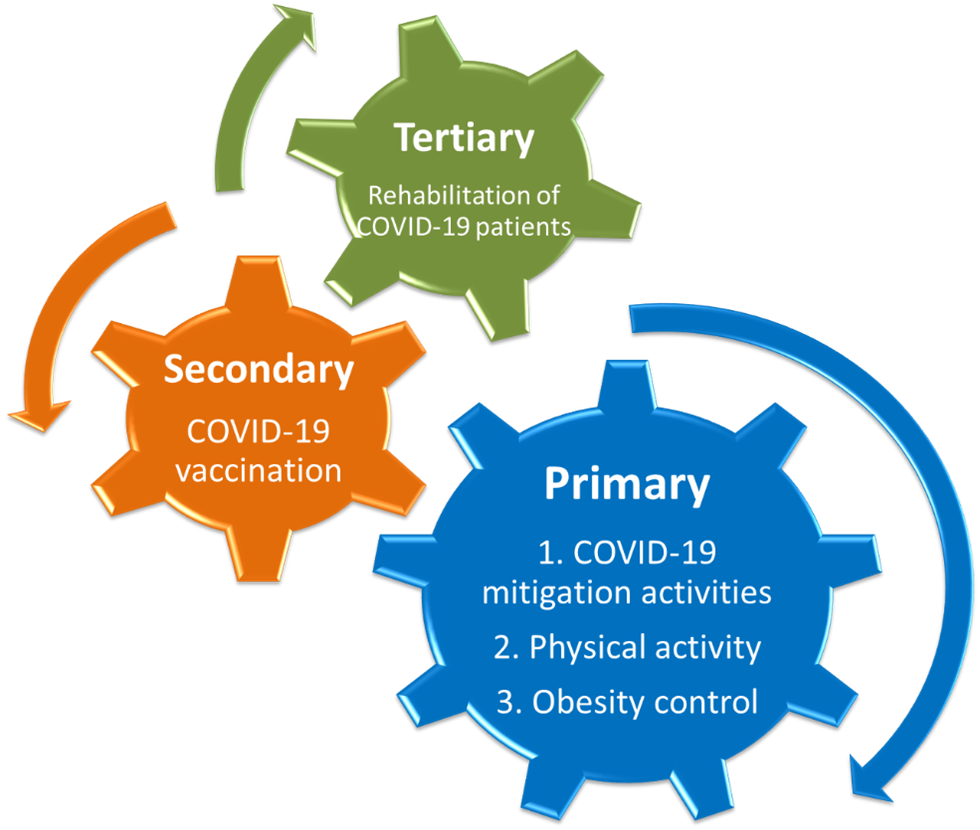
Figure 2. Protective role of regular physical activity against respiratory infections. Risk for bacterial and viral infectious disease mortality was more than 50% lower as determined in a cohort of 97,844 adults followed for over nine years (8).
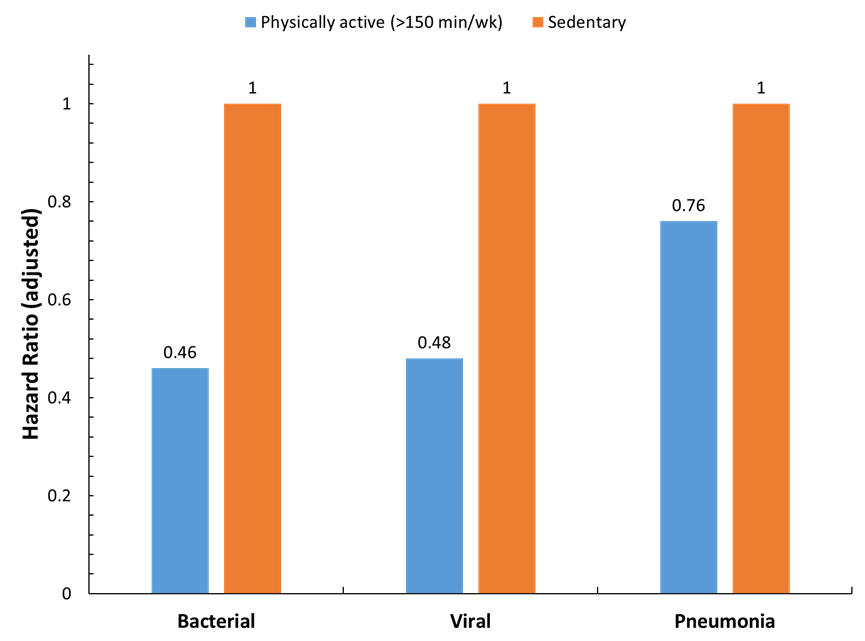
Figure 3. Immune responses of individuals with typical (A) compared to severe (B) COVID-19. SARS-CoV-2 is unusually effective in evading the activation of early innate immune responses (e.g., IFNs), and the virus can multiply more quickly before triggering an appropriate adaptive immune response. In a typical COVID-19 infection, the innate immune system responds early and strongly to SARS-CoV-2 and primes T cells from the adaptive immune system to reduce the viral load. This response, however, can be atypical and amplified with age because of a smaller naïve T cell pool, and with obesity because of dysfunctional T cells. The innate response may be excessive (in response to diminished adaptive immunity) leading to high levels of inflammation and damage in multiple tissues including the lungs (20).